

















Dream Sock®
FDA-cleared smart baby monitor for safer sleep and trusted reassurance
Buy now and pay as low as
$26/mo with

Color:
- Dream Sock® Sensor
- 4 Fabric Socks (fits babies 1-18 months, 6-30 lbs)
- Base Station
- Free Access to Owlet Dream App
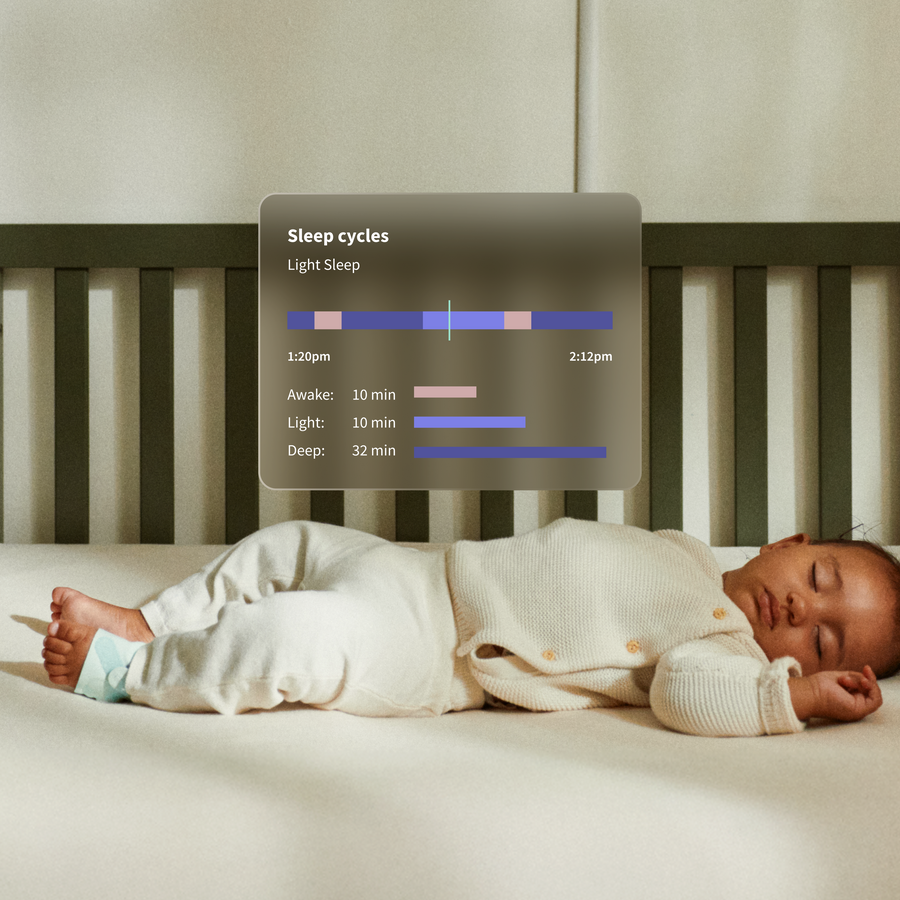
Better sleep for your baby. Better rest for you. Professional-level monitoring and actionable insights that help you sleep well, understand patterns, and feel confident in your baby’s wellness - not just when something happens. Dream Sock goes beyond traditional monitors to give you the wellness and sleep insights you really need. Medical-grade monitoring gives you real-time visibility into your baby’s wellness, so you know, not guess. Track live pulse rate, oxygen levels, sleep and trends overtime so you can understand sleep, wellness trends, and daily rhythms.
- Device Support: iOS 14+, Android 7+
- Network Support: 2.4GHz Wi-Fi
- Connectivity: Wi-Fi + Bluetooth
- Bluetooth Operating Range: 40-100 ft
- Charging Type: Wireless Base Station
- Power Source: USB-C (wall adapter included)
- Sensor Type: Pulse Oximeter
- Battery Life: Up to 16 hours; 8-hour charge in 20 min; full in 90 min
- Battery Type: Rechargeable Lithium-Ion
- Materials: Spandex + medical-grade sensor
- FDA-Cleared (De Novo clearance)
- Medical-Grade Pulse Oximeter (Over-the-Counter)
- Clinically Tested for Accuracy
- Clinically validated across all skin tones and through gentle motion
- Industry Certifications: FCC Certified, CPSC Compliant
- Built with anti-hacking protection: 256-bit encryption + elliptic curve authentication
The first FDA-cleared smart baby monitor of its kind. Real-time health insights, so you can rest easier.

"As a first-time mom with postpartum anxiety, I was constantly checking on my baby. Our Dream Sock helped me rest between feeds and stay calm during the night."
Kylee W.
verified user

"Been using it for 5 1/2 months and it’s my #1 recommend baby registry product!! "
Em
verified user

"We use our Dream Sock every single night. It has done nothing but provide us with the insight and information to ensure our baby is okay and is sleeping peacefully."
Breanna Z.
verified user
Explore Features

Medical-grade accuracy, at home
- FDA-Cleared Dream Sock is the first and only FDA-cleared smart baby monitor of its kind, using the same pulse oximetry technology trusted by hospitals.
- Real-Time Health Insights You’ll receive live pulse rate and oxygen readings, as well as wakings and sleep trends, all with accuracy you can trust.
- Trusted Reassurance For More Confidence From Day One. Dream Sock has been clinically evaluated for SpO2 accuracy across all skin tones under motion and non-motion conditions, demonstrating accuracy within +/- 3% of gold-standard arterial blood gas measurements.

Know When Your Baby Needs You Most
- Peace of Mind, at a Glance The included bedside Base Station glows green when your baby’s readings are within preset ranges, so you can rest easy without constantly checking your phone.
- Oxygen Notifications If your baby’s oxygen level dips below the preset zone, the Base Station will flash red and sound an alarm to wake you, while the Owlet Dream App sends an alert straight to your phone.
- Pulse Rate Notifications Dream Sock also monitors your baby’s pulse rate. If it rises too high or falls too low, you’ll be notified on the Base Station and in the Owlet Dream App in real time.

Build better sleep habits.
Consider Owlet your partner in sleep insights. Through the Dream App, you can learn your baby's unique sleep patterns and receive personalized insights that will help you understand your baby's needs for rest and create better habits from day one.

One App, Instant Confidence & Reassurance
- Sleep & Health Summarized Open the Owlet Dream App and instantly see your baby’s live readings and sleep metrics on the home screen to help you feel reassured right away.
- Nightly History & Trends Dive deeper into your baby’s sleep and health patterns with detailed charts from overnight or past nights. Spot trends, adjust routines, or share valuable data with your pediatrician.
- Smarter Sleep Schedules With Predictive Sleep, the app learns your baby’s natural rhythms and guides you on the best times for naps and bedtime.
Trusted Monitoring When It Matters Most
Monitor your baby’s pulse rate and oxygen in real-time with clinical accuracy, using hospital-trusted pulse oximetry and medical-grade technology you can rely on.
Comfort Engineered for Baby
Soft, snug, and rigorously tested for accuracy on every skin tone and through gentle movement. It delivers continuous health monitoring without interfering with comfort or natural sleep.
Know When Your Baby Truly Needs You
Real-time alerts tell you exactly when health readings leave the preset zones, not just noise or movement, so you respond with confidence, not guesswork, every night.
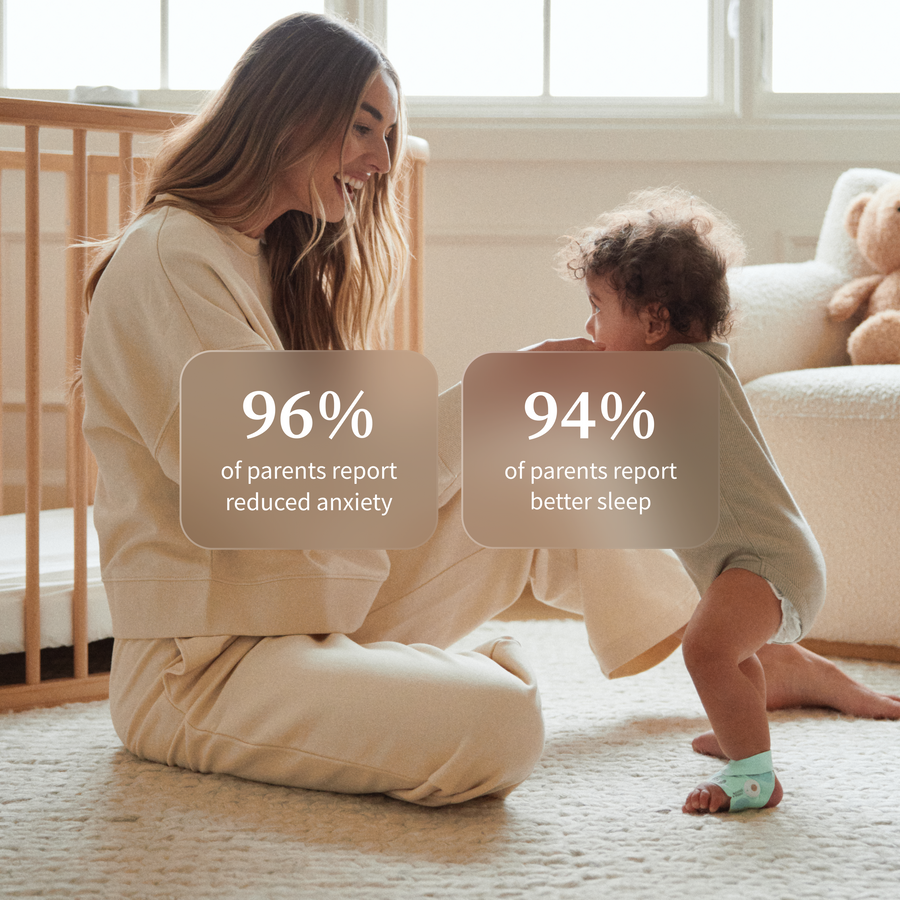
Supported and Better Sleep
- 96% of parents report reduced anxiety
- 94% of parents say they sleep better
- 75% of parents feel less stress
Insight when you want it. Calm when you need it.
Dream Sock vs. Traditional Monitors
WHAT'S INCLUDED
Everything to Start Monitoring From Night One
Frequently Asked Questions
Is Dream Sock safe and accurate?
Yes. Dream Sock is safe and accurate when used as intended. Achieving FDA De Novo clearance means Dream Sock was clinically tested and evaluated for oxygen saturation (SpO2) accuracy across all skin tones (Type I-VI on the Fitzpatrick scale) under motion and non-motion conditions. Dream Sock has been demonstrated to be accurate within +/- 3% of gold-standard arterial blood gas measurements.
Does Dream Sock require a subscription?
No subscription is required to use Dream Sock. Once purchased, you’ll have full access to live health readings, historical data, and notifications through the Owlet Dream App.
Why does FDA clearance matter?
FDA clearance means Dream Sock underwent rigorous clinical testing in both home and hospital environments and was proven to be as accurate as medical-grade pulse oximeters labeled for use in infants. Dream Sock is currently the only baby monitor on the market that is FDA cleared. While it isn’t a replacement for medical care, it gives parents trustworthy, real-time insights into their baby’s well-being.
Is Dream Sock eligible for HSA/FSA or other flexible payment options?
We understand that Dream Sock is an investment in your peace of mind. That’s why we offer flexible payment plans through partners like Affirm and Klarna. Dream Sock is also eligible for purchase with HSA/FSA funds. We also recommend signing up for our email list here to be the first to know about special promotions or discounts.
Is Dream Sock a medical device?
Yes. Dream Sock is a Class II medical device cleared by the FDA for over-the-counter use. Achieving FDA clearance means Dream Sock was clinically tested in both home and hospital environments, and proven to be as accurate as medical-grade pulse oximeters labeled for use in infants. While it does not replace medical care, it empowers parents with valuable insights to support their baby’s well-being at home.
















